- Research & Instruments
- How we study - Our instruments and methods
- Instruments and Methods
- On Land
- Nanoscale Secondary Ion Mass Spectrometer (NanoSIMS)
Nanoscale Secondary Ion Mass Spectrometer (NanoSIMS)
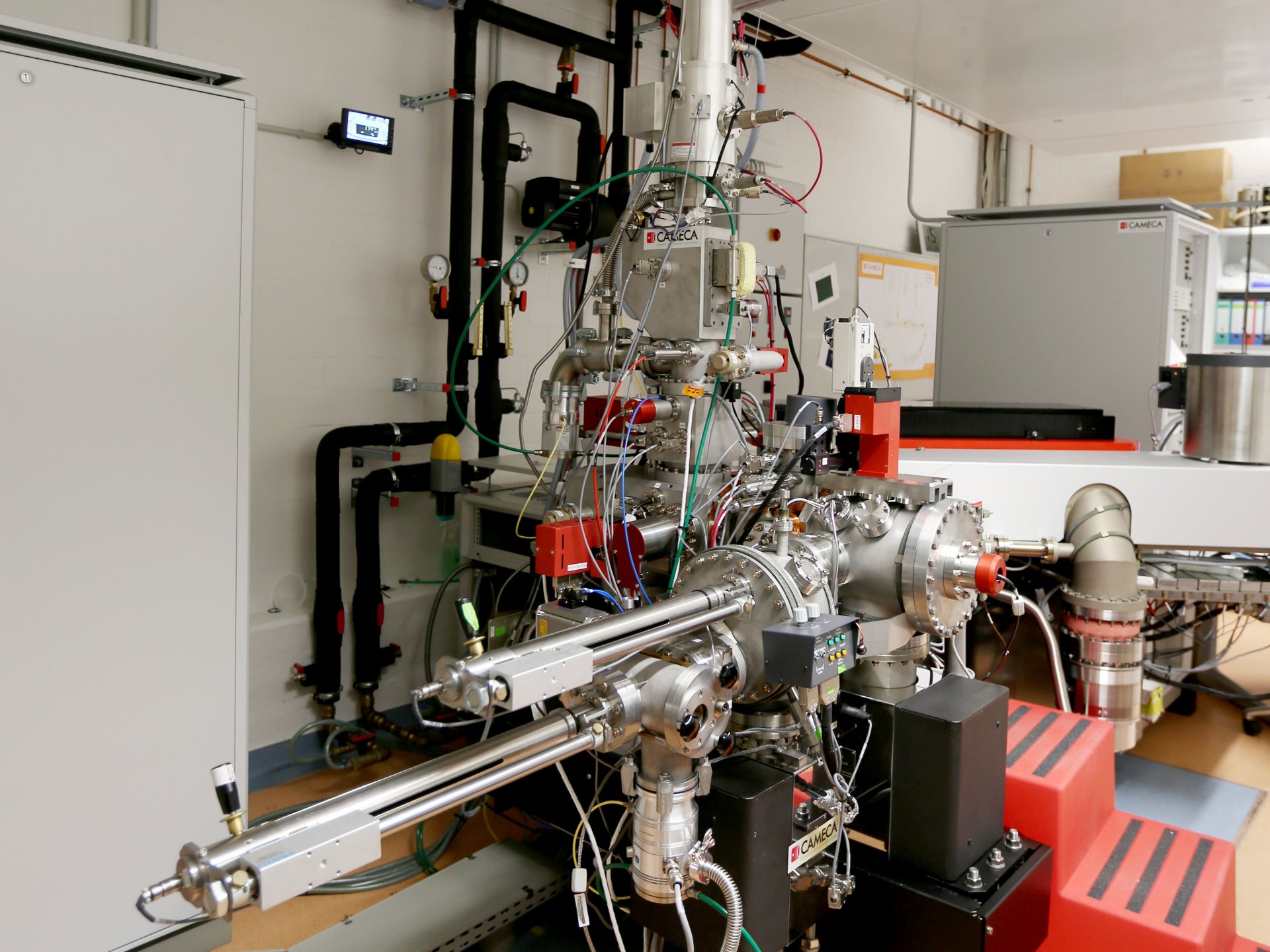
What can the NanoSIMS do?
NanoSIMS stands for “Nanoscale Secondary Ion Mass Spectrometer”. It is a mass spectrometer with special optics that create enormous spatial resolution: We can observe things that are only about 50 nm in size. For example, we can study the structures and processes in a single cell and “watch it at work”. There are only about 50 such devices worldwide. Ours was one of the first that was used to answer microbiological questions.
At the MPI Bremen, the NanoSIMS is used mainly for the elemental and isotopic analysis of microbiological samples from different environments. With this method, it is possible to analyse the metabolism of microbes. To achieve this, environmental samples are incubated with chemical elements such as carbon or nitrogen (i.e. nutrients for microorganisms) that are isotopically marked. Through microbial activity, these heavy isotopes are incorporated and enriched in the microbial cells. These enrichments can be measured using the NanoSIMS. This allows us to precisely quantify the metabolic activity of individual cells.
Prior to NanoSIMS analysis, the different microbes can be identified using various methods such as CARD-FISH, which fluorscently marks the targeted cells. This information allows linking a cell's to its activity and thus identifies the role of different micro-organisms in an ecosystem.
How does NanoSIMS work?
Secondary ion mass spectrometers (such as the NanoSIMS at our Institute) use a finely focused primary ion beam (in our case cesium or oxygen) to ablate and ionize a small part of the sample surface. In this process, the primary ion beam can be focused on a very small spot down to a beam size of 50–150 nm.
Afterwards, the secondary ions formed by the impact of the primary ion beam are introduced into a mass spectrometer and separated according to mass. The high mass resolution of the mass analyser makes it possible to even separate interferences (superpositions) of different ions with the same mass. For example, thanks to the high mass resolution, we can differentiate the 32 isotope of sulphur (32S) from the 16O2 molecule, although both have mass 32. In the multi-collection system, these individual ions can then be counted. Thanks to the high sensitivity of the instrument, extremely small sample quantities can be analysed. The frequency of the individual ions arriving at the various detectors can then be visualized in distribution images.
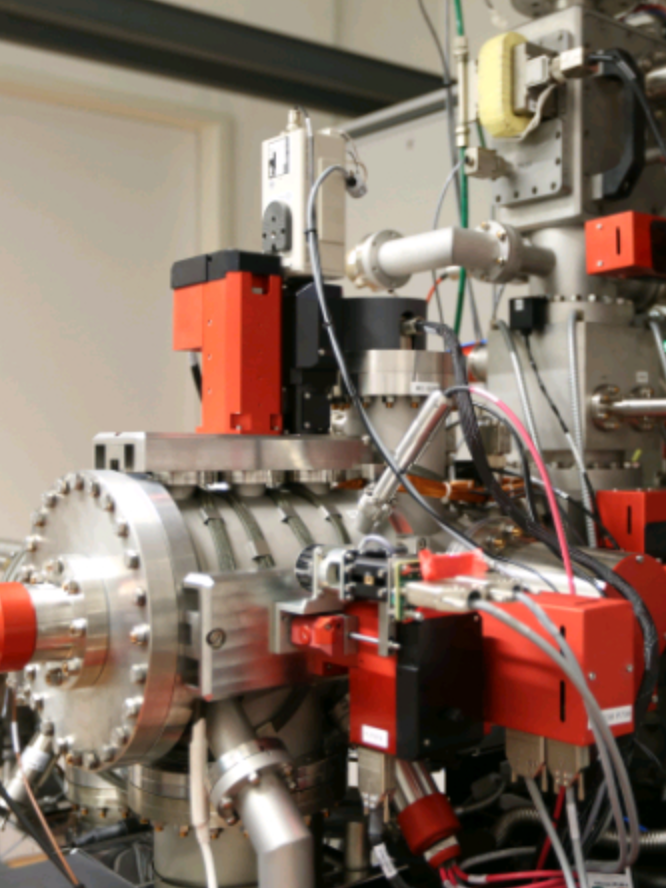
The Equipment of our NanoSIMS
The MPI in Bremen is equipped with a NanoSIMS 50L from CAMECA. This is a secondary ion mass spectrometer (SIMS) optimized for high lateral resolution in the range of 50–150 nm (nanoscale). It is equipped with two different ion sources for primary beam generation. To measure negatively charged secondary ions (e.g. carbon, nitrogen, sulfur, and phosphorus), we use a cesium ion source.
Our instrument is also equipped with a radio frequency plasma oxygen ion source for measuring positively charged secondary ions. A multi-collection system with seven detectors is used to detect the secondary ions. Each detector position is equipped with a Faraday detector and an electron multiplier.
The NanoSIMS in action
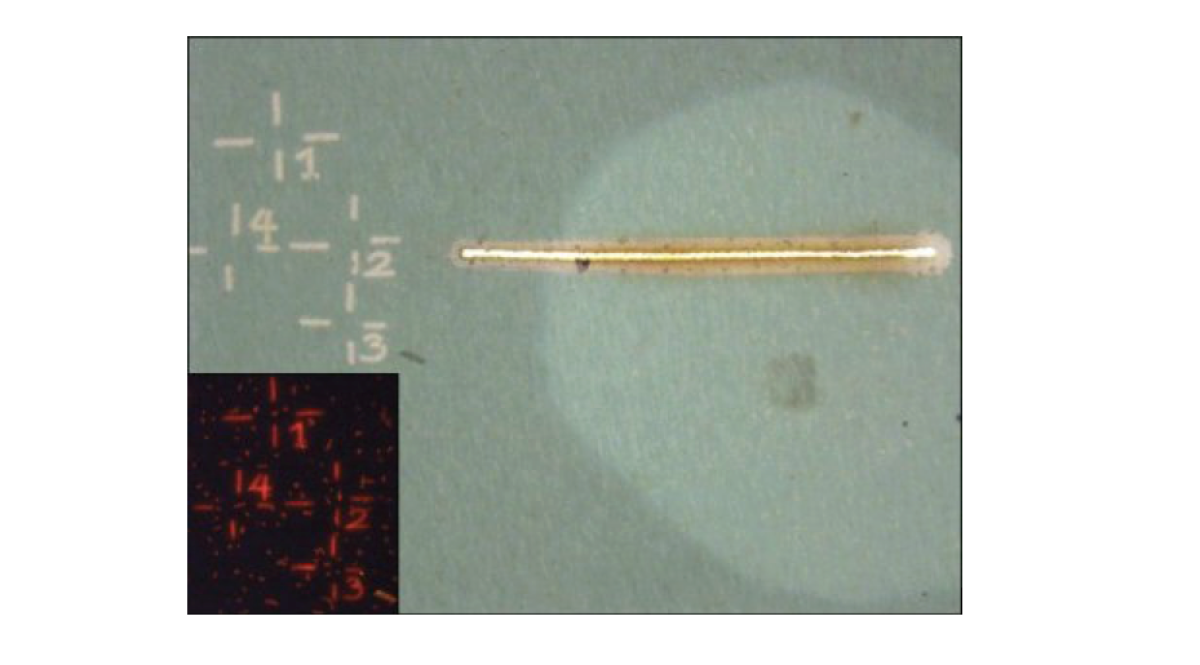
Preparation of a sample with the LMD
Before an analysis can be carried out with the NanoSIMS, the areas with cells of interest must be marked. This is done with a laser microdissection microscope (Leica LMD 6500). The markings facilitate orientation during the analysis with the NanoSIMS.
Our laser dissection microscope is equipped with different objectives (10x – 63x) and fluorescence filters (e.g. DAPI, Cy3, Cy5, or Alexa594) in order to identify, mark, and even dissect cells in a sample. These markings are done using a UV laser (wavelength: 355 nm, pulse frequency: 80 Hz, pulse length: < 4 ns, average pulse energy: 70 μJ). Images of marked areas are taken with a CC7000 color camera.
Read more about her: https://link.springer.com/protocol/10.1007/978-1-0716-1115-9_13
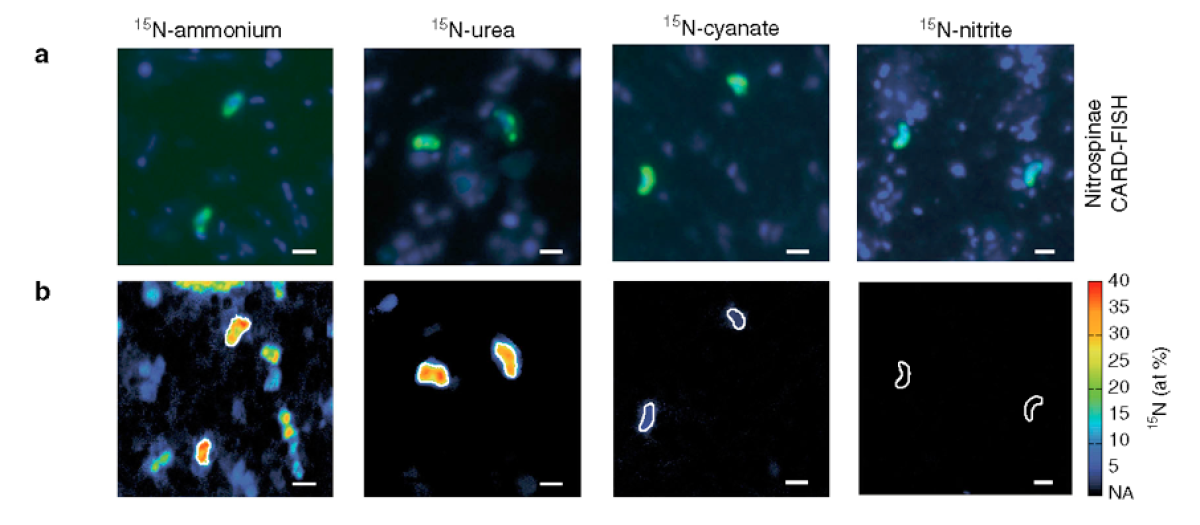
a) Representative CARD-FISH images of Nitrospinae (green, stained with probe Ntspn759) and other cells (blue, stained with DAPI).
b) Corresponding NanoSIMS image of 15N at% enrichment after addition of 15N-ammonium, -urea, -cyanate, or -nitrite. Nitrospinae are characterized by white outlines. The scale bar is 1 μm in all images. (Max Planck Institute for Marine Microbiology in: Nature Communications, 2020, doi: 10.1038/s41467-020-14542-3)
Insight into the life strategies of two groups of nitrifying micro-organisms
Nitrification is the conversion of the nitrogen compound ammonia to nitrite and then to nitrate. In the sea, the first step, ammonia oxidation to nitrite, is carried out by ammonia-oxidising archaea. The second part, the transformation of nitrite to nitrate, is carried out by nitrite-oxidising bacteria, especially Nitrospinae. However, there are ten-fold more of the ammonia-oxidising archaea than of Nitrospinae.
In order to explain this difference in abundance, researchers at MPI Bremen investigated the biomass of the micro-organisms as well as the growth rates and activity of individual cells using - among other techniques - NanoSIMS. They showed that the Nitrospinae are significantly more active and grow much faster than the ammonia-oxidising archaea. Nitrospinae are thus clearly more efficient than the Archaea.
The researchers also used the NanoSIMS to investigate which nitrogen compounds the partners ammonia-oxidising archaea and Nitrospinae use for their cell growth. They observed that while the archaea almost exclusively use ammonium, Nitrospinae mainly use organic nitrogen, namely urea and cyanate.
Further details on the topic can be found in the press release: "Mystery of marine recycling squad solved”
The corresponding publication in Nature Communications is available here: https://www.nature.com/articles/s41467-020-14542-3
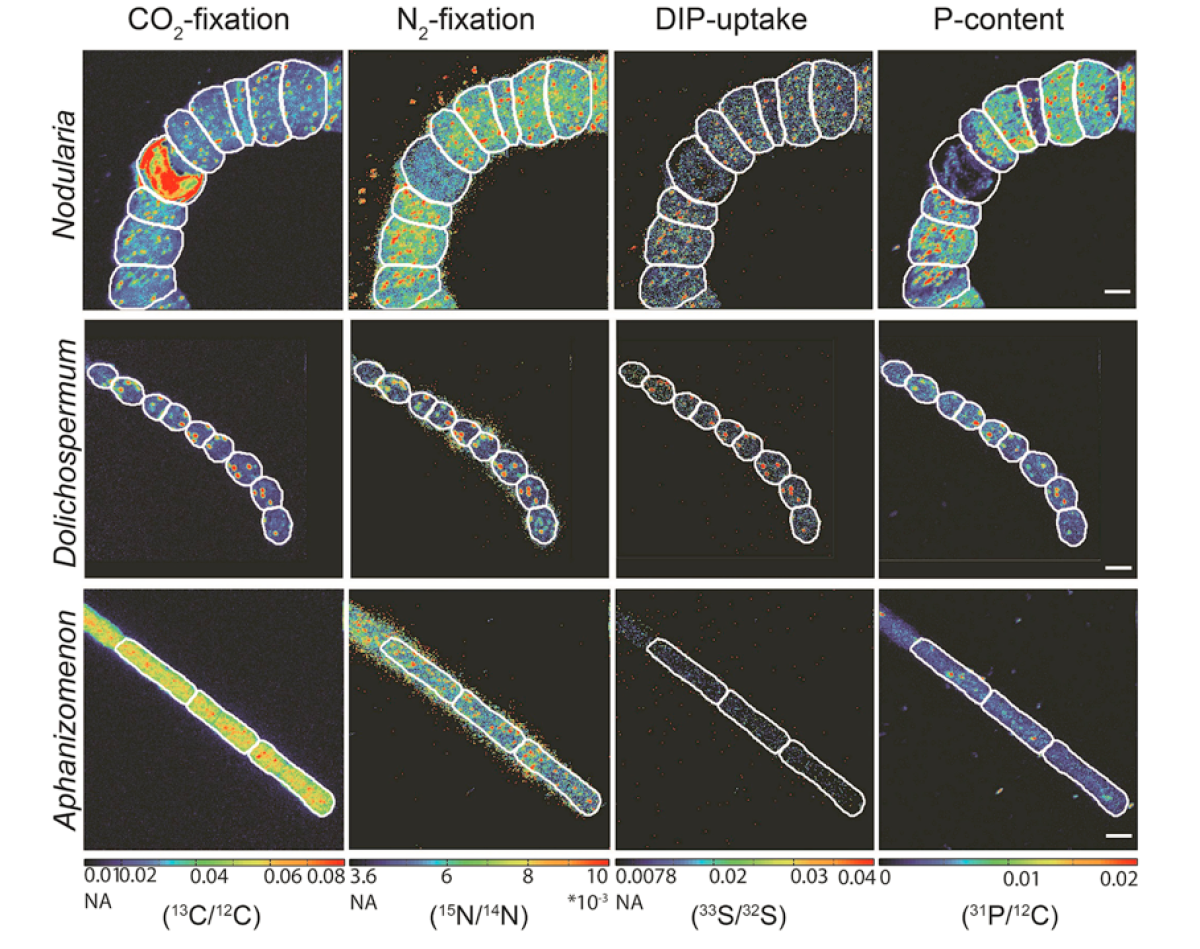
Visualization of phosphorus uptake in various cyanobacteria from the Baltic Sea
Phosphorus is essential for the cellular function of microorganisms. We were able to develop a method based on NanoSIMS analytics that allows the quantification of phosphorus uptake by single cells in combination with cellular CO2and N2 fixation. The uptake of radioactive 33P into the cell and the subsequent decay of 33P to 33 Scan be used to visualize the inorganic phosphorus uptake in the cells via the change in the 33S/32S ratio.
This development made it possible to show the influence of phosphorus on the growth of various cyanobacteria species in the Baltic Sea. It was shown that phosphorus availability in the Baltic Sea can play a critical role in the development of Nodularia blooms (which form harmful algal blooms, HABs).
The corresponding publication in Scientific Reports is available here: https://www.nature.com/articles/s41598-018-35310-w
Overview of research projects from the Department of Biogeochemistry in which the NanoSIMS is regularly used:
Who uses the NanoSIMS?
The NanoSIMS is used by many members of the Department of Biogeochemistry. Other researchers at the Institute can also use the device if necessary. External scientists can also gain access. If you are interested, please contact below.
Please direct your queries to
Scientist
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
3130 |
|
Phone: |

Technician
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
3131 |
|
Phone: |
