- Research & Instruments
- How we study - Our instruments and methods
- Instruments and Methods
- On Land
- Environmental scanning electron microscope
Environmental scanning electron microscope
What is a environmental scanning electron microscope (SEM)?
When examining and imaging minute objects such as marine micro-organisms, we quickly reach the limits of optical microscopy. In order to be able to image these diverse organisms, we must use scanning electron microscopy instead. In the Biogeochemistry working group, we have a scanning electron microscope (SEM) for this purpose. It is equipped with various detectors for image generation and analyses.
How does a scanning electron microscope work?
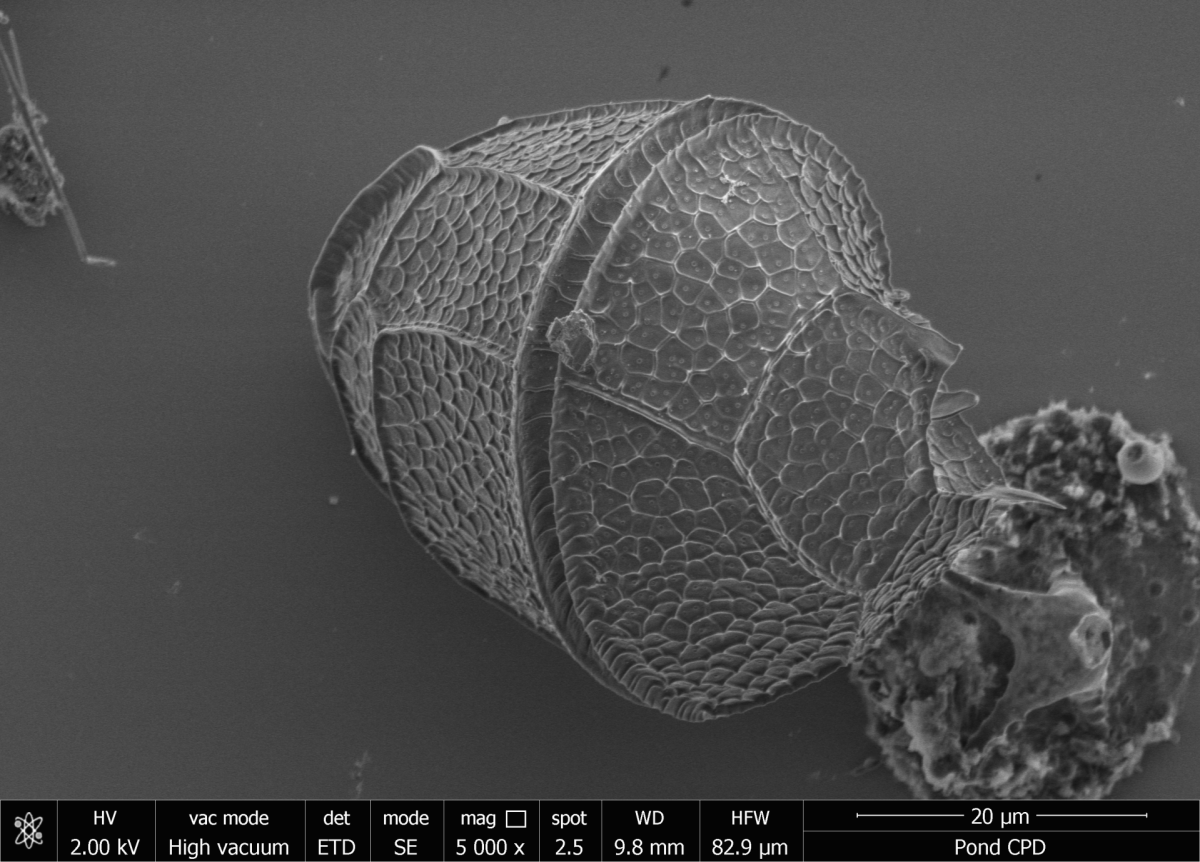
An SEM allows a higher resolution (down to 1 nm at 30 kV in high vacuum) and depth of field than a light-optical microscope. A field emission cathode generates an electron beam that is focused on the sample by electromagnetic lenses. The surface of the sample is then scanned point by point by this electron beam in a rectangle. Various acceleration voltages (500 V to 30 kV) can be used for the electrons. When the electron beam (primary electrons, PE) strikes the surface of the sample, various interactions occur with the atoms of the sample, and various signals are created – including secondary electrons (SE), which can be used for imaging.
Because the SE are low-energy electrons, they originate only from a thin layer from the surface of the sample. SE images thus contain information about the topography contrast. In order to be able to see fine structures in minute micro-organisms, a gentle and three-dimensional, structure-preserving sample preparation with critical point drying (CPD) is required. A CPD device is available for this purpose.
Another signal, the back scattered electrons (BSE), are higher in energy than the SE. BSE images contain information about the composition of the sample through the material contrast. Elements with higher nuclear charge numbers appear brighter on the image than elements with lower nuclear charge numbers.
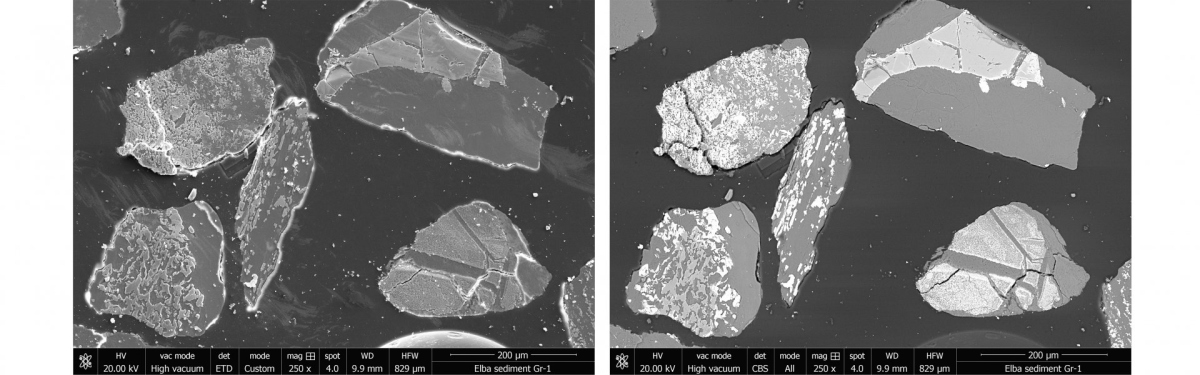
The SEM is equipped with different detectors so that SE and BSE can be detected in all vacuum working ranges. Depending on the nature of the sample, investigations can be carried out in a high vacuum (6 × 10−4 Pa), low vacuum (10–130 Pa), and ESEM mode (for liquids, 10–4000 Pa).
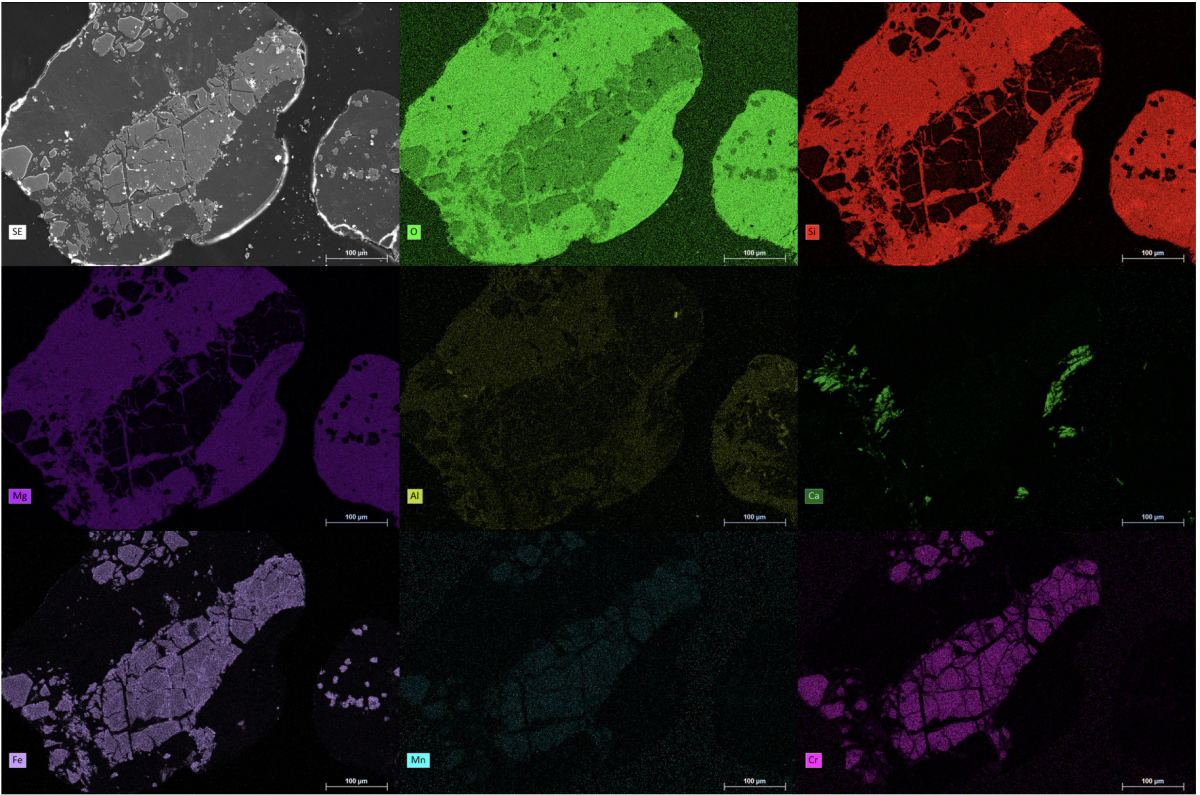
X-ray radiation also arises from the interaction of the PE with the atoms of the sample. This can be used for the chemical analysis of a sample because each chemical element has a characteristic X-ray radiation. For the detection of the characteristic X-rays, our SEM is equipped with a double detector system from Bruker Nano Analytics for energy dispersive X-ray spectroscopy (EDS or EDX). The EDS system has two Xflash 6/30 detectors (30 mm2 detector area) with an energy resolution < 123 eV for the manganese Kα-spectral line. The data is evaluated using the Bruker Esprit 2.1 software. Both point analyses and element mapping can be carried out with this system.
The SEM can also be used for scanning transmission electron microscopy (STEM). The sample is imaged and analysed by means of transmitted electrons. Thin sections as low as 100 nm thick can be examined with this method. For this purpose, a special detector (STEM detector) is placed underneath the sample. Accelerating voltages between 20 and 30 kV are generally used.
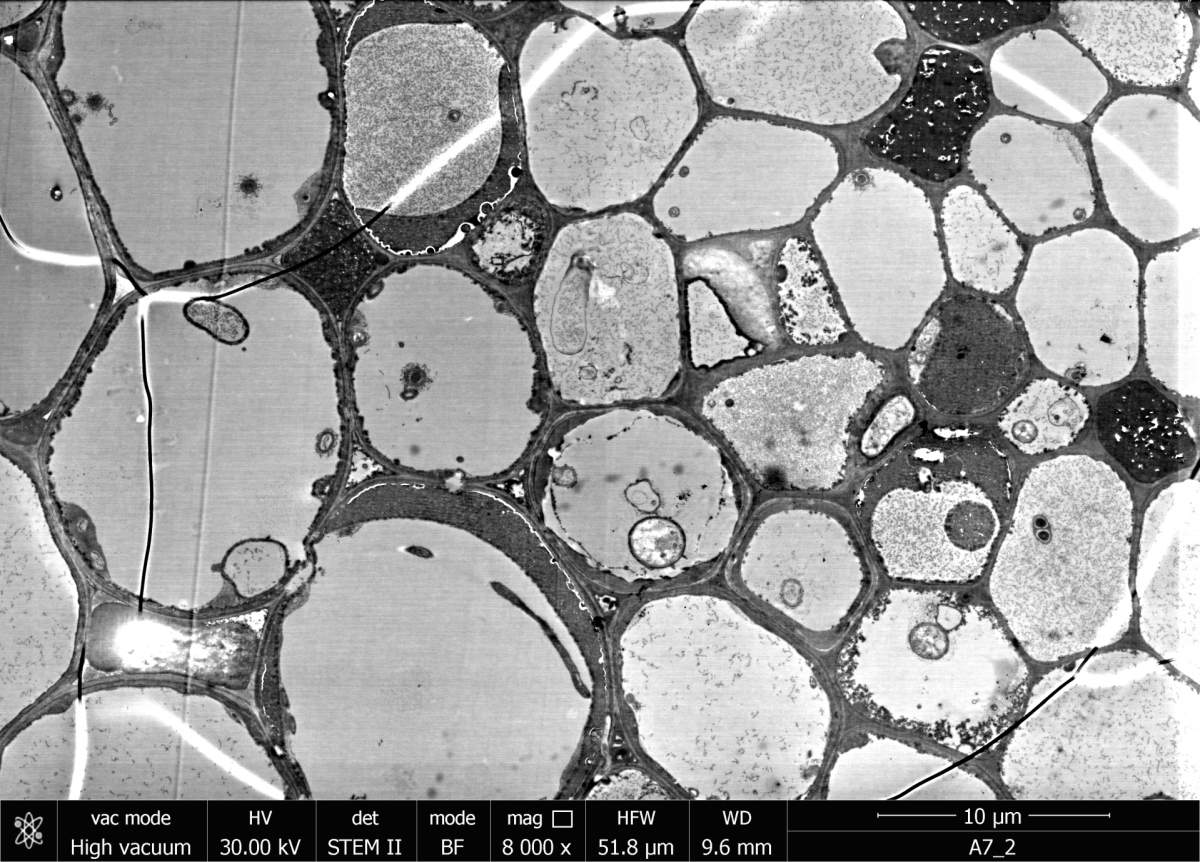
The SEM in the Department of Biogeochemistry at the MPI Bremen can also be equipped with a SECOM platform. SECOM enables correlative imaging of a sample using scanning electron and fluorescence microscopy. For this, the sample must be prepared on a glass slide coated with indium tin oxide (ITO). Scanning electron microscopy is performed on the conductive top side (ITO) of the glass slide and fluorescence microscopy from the bottom side through the glass slide. This makes it possible to identify certain micro-organisms by fluorescence and to describe surface structures using SEM.

The scanning electron microscope in action
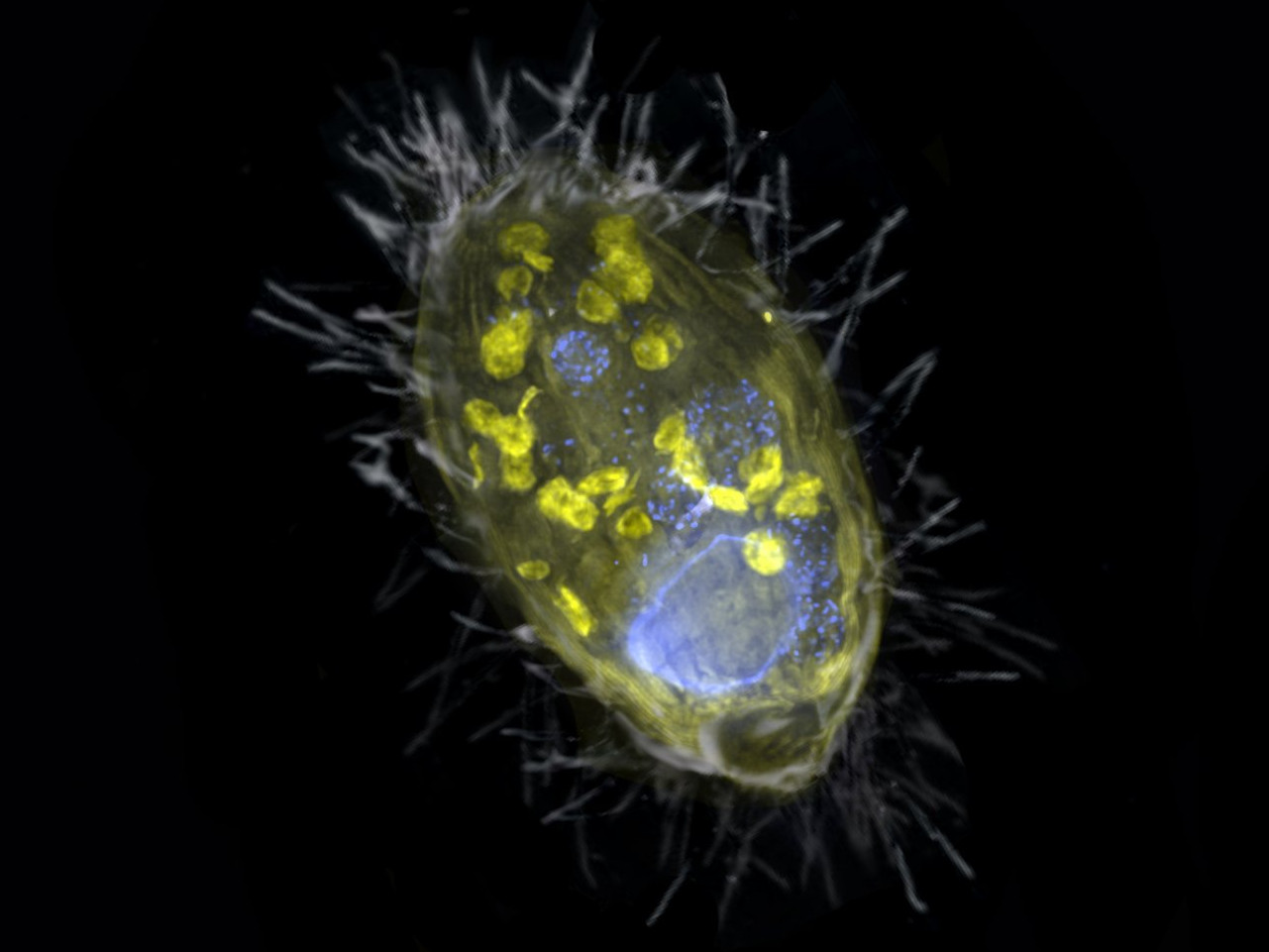
New form of symbiosis discovered
They are also called power plants of the cells: the mitochondria. They are present in almost all eukaryotic cells and they supply the cells with energy. Until now, it was assumed that only mitochondria can act as the cells’ energy providers. Scientists at the Max Planck Institute for Marine Microbiology have now discovered that symbiotic bacteria can fulfil this function too. Their findings shed a completely new light on the survival of simple eukaryotes in oxygen-free environments.
The scientists have found a unique bacterium that lives inside a unicellular eukaryote and provides it with energy. Unlike mitochondria, this so-called endosymbiont derives energy from the respiration of nitrate, not oxygen. Such partnership is completely new.
For this study, also the scanning electron microscope was used.
You can find more informatin about the study in the press release "New form of symbiosis discovered"
Here you find the original publication:
Jon S. Graf, Sina Schorn, Katharina Kitzinger, Soeren Ahmerkamp, Christian Woehle, Bruno Huettel, Carsten J. Schubert, Marcel M. M. Kuypers, Jana Milucka: Anaerobic endosymbiont generates energy for ciliate host by denitrification. Nature, 2021
Who uses the scanning electron microscope?
It is mainly used by scientists of the Department of Biogeochemistry. However, it is also open to all other scientists of the institute as well as to external researchers in the context of collaborative projects.
Contact
Scientist
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
3130 |
|
Phone: |