- Press Office
- Press releases 2020
- Sugar turns brown algae into good carbon stores
Sugar turns brown algae into good carbon stores
You may like them or not, but almost everyone knows them: brown algae such as Fucus vesiculosus, commonly known as bladderwrack, grow along the entire German coast. Giant kelp like Macrocystis or Sargassum grow closely together along the coasts but can also form floating aggregates that can cover the Atlantic from west to east. Some ecologists see this this very productive ecosystem as a marine counterpart to rainforests on land. In these algal forests, large amounts of carbon dioxide are stored, making them an important part of the global carbon cycle.
Andreas Sichert from the Max Planck Institute for Marine Microbiology dedicated his PhD to the question how brown algae can be such a good sink of carbon: “Main constituents of algal biomass are their cell walls – a tight network of proteins and long-chained sugars. When the algae die, we actually have little clue about the fate of algal biomass in the ocean, for example which compounds are degraded fast or slowly”.
Firm and flexible
The Atlantic coast is not a cozy habitat. Tides, wind and waves demand special adaptations from the inhabitants of this harsh environment. Brown algae developed a special cell wall structure, making them both firm and flexible, and enabling the plant to successfully withstand heavy currents and waves. A major component of the cell walls is the polysaccharide fucoidan, a long-chained sugar accounting for about a quarter of algal dry mass. Likely, fucoidan can regulate the water content of the cell wall which protects brown algae from drying out at low tide.
What role this sugar plays in the long degradation process of brown algae was analyzed by scientists from the research group Marine Glycobiology at the Max Planck Institute for Marine Microbiology and the MARUM, Center for Marine Environmental Sciences at the University of Bremen. For their study, they cooperated with colleagues from the Massachusetts Institute of Technology, from the University of Greifswald and from the University of Vienna. “It was already known that microbial communities hydrolyze fucoidan slower than other algal polysaccharides and thus fucoidan might act as carbon sink” says Andreas Sichert from the Max Planck Institute for Marine Microbiology, first author of the study, published in the scientific journal Nature Microbiology in May 2020. “Usually, polysaccharides are a favorite energy source for bacteria, but the reason why fucoidan should be barely digestible remained unclear”.
Only specialists degrade this sugar
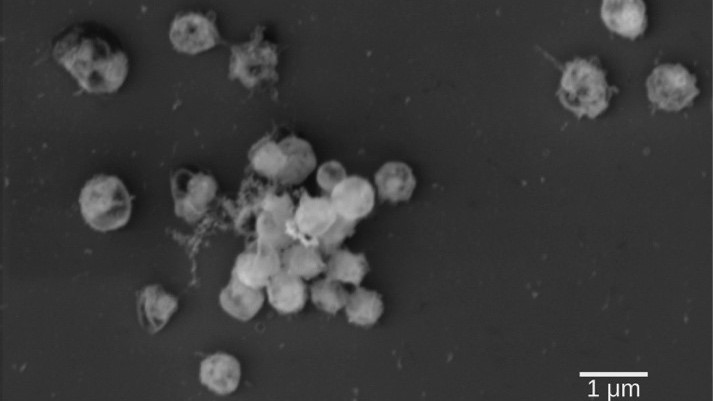
So far, the fucoidan degradation pathways were only partially known, but it was evident that they involve a substantial number of enzymes either distributed within a microbial community or housed within individual, highly specialized bacteria. The scientists from Bremen examined the latter theory and analyzed newly isolated bacteria of the genus Lentimonas, belonging to the phylum Verrucomicrobia. Even the isolation of these Lentimonas bacteria was challenging. “From initially more than thousand colonies, only one was able to degrade fucoidan in the end,” remembers Christopher H. Corzett from the Massachusetts Institute of Technology, first author of the study next to Andreas Sichert.
“We could show that Lentimonas acquired a remarkably complex machinery for the degradation of fucoidan that uses about one hundred enzymes to liberate the sugar fucose – a part of fucoidan”, says Jan-Hendrik Hehemann, leader of the research group Marine Glycobiology. „This is probably one of the most complicated biochemical degradation pathways for natural material that we know of.” Fucose is then metabolized via a bacterial microcompartment, a proteinaceous shell that shields the cell from the toxic intermediate lactaldehyde. „The need for such a complex catabolic pathway underpins the recalcitrance of fucoidans for most marine bacteria and it shows that only highly specialized organisms in the ocean are able to break down this algal sugar,“ says Hehemann. „This can explain the slower turnover of the algal biomass in the environment and suggests that fucoidans sequester carbon in the ocean.“
Potential for pharmacology
Scientists are also interested in enzymes for fucoidan degradation because it may be a pharmacologically active molecule that shows similar effects to heparin in blood clotting. “Enzymes that specifically fragment fucoidan and thus help to characterize its structure are of great scientific interest because they enable researchers to understand the effects of fucoidan and to open up these marine sugars for biotechnological applications,” says Thomas Schweder, participating microbiologist from the University of Greifswald.
Original publication
Andreas Sichert#, Christopher H. Corzett#, Matthew S. Schechter, Frank Unfried, Stephanie Markert, Dörte Becher, Antonio Fernandez-Guerra, Manuel Liebeke, Thomas Schweder, Martin F. Polz, Jan-Hendrik Hehemann: Verrucomicrobia use hundreds of enzymes to digest the algal polysaccharide fucoidan. Nature Microbiology, May 2020
DOI: 10.1038/s41564-020-0720-2
# both authors contributed equally
Funding
This study was funded by the DFG as part of the Emmy Noether group “Resolve” and the research group FOR2406 “POMPU - Proteogenomics of marine Polysaccharide Utilization”.
Participating institutions
- Max Planck Institute for Marine Microbiology, Bremen, Germany
- MARUM – Center for Marine Environmental Sciences, Bremen, Germany
- Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, USA
- University of Greifswald, Germany
- University of Vienna, Austria
Please direct your queries to:
Group leader
MARUM MPG Bridge Group Marine Glycobiology
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Room: |
2126 |
|
Phone: |

Contact person at the University of Greifswald:
Prof. Dr. Thomas Schweder
Pharmaceutical biotechnology
Institute for Pharmacy
Telefon: +49 3834 420 4212
E-Mail: [Bitte aktivieren Sie Javascript]