- PhD Thesis - Summary
PhD Thesis - Summary
The microbial Party – Electro, Hard Rock, and Heavy Metal
How geomicrobiology helps us to understand climate changes.
* This article is more detailled in German*
Climate change, greenhouse gases and their effects
Understanding climate changes – this is one of the great challenges for humankind in the 21st century. Greenhouse gases (GHGs) such as nitrous oxide (N2O) are one of the main drivers of climate changes. Besides agricultural farmlands, coastal sediments were identified as an additional significant N2O sources. In iron-containing coastal sediments bacteria can form N2O during the degradation of nitrate. So-called iron-cycling bacteria (FeB) occur in complex communities and can metabolize iron minerals ("heavy metal" & "hard rock"). Until now, the contribution of the abiotic processes - besides microbial mediated processes - to the global N2O budget was unknown. My research study is the first worldwide that has quantified the geochemical formation of N2O by iron in coastal sediments. These findings will help to improve our understanding of climate changes and its consequences.
Coastal sediments as important N2O source
Some key factors of climate changes are already known (solar cycles, precision of the Earth's axes, etc.). In addition, the formation of GHGs is one of the most important origins of climate changes. For example, GHGs such as carbon dioxide (CO2), methane, ozone, and N2O can cause great damage in the stratosphere. In 1998, coastal marine habitats were identified as important N2O sources. Sandy sediments are representative of about 70% of all global shelf sediments and are expected to release up to 1.9 teragrams of N2O-N per year. In the future, the release of N2O could even increase further due to the extensive use of nitrate fertilizers. Rainfall can wash out the nitrate from the farmland, which then deposits in the nearby sediments. The activity of nitrate-degrading bacteria living in these sediments and abiotic reactions can form N2O. This leads to the central research question: To what extent do microbial and abiotic processes contribute to the formation of N2O in coastal sediments?
Biogeochemistry of iron-containing sediments
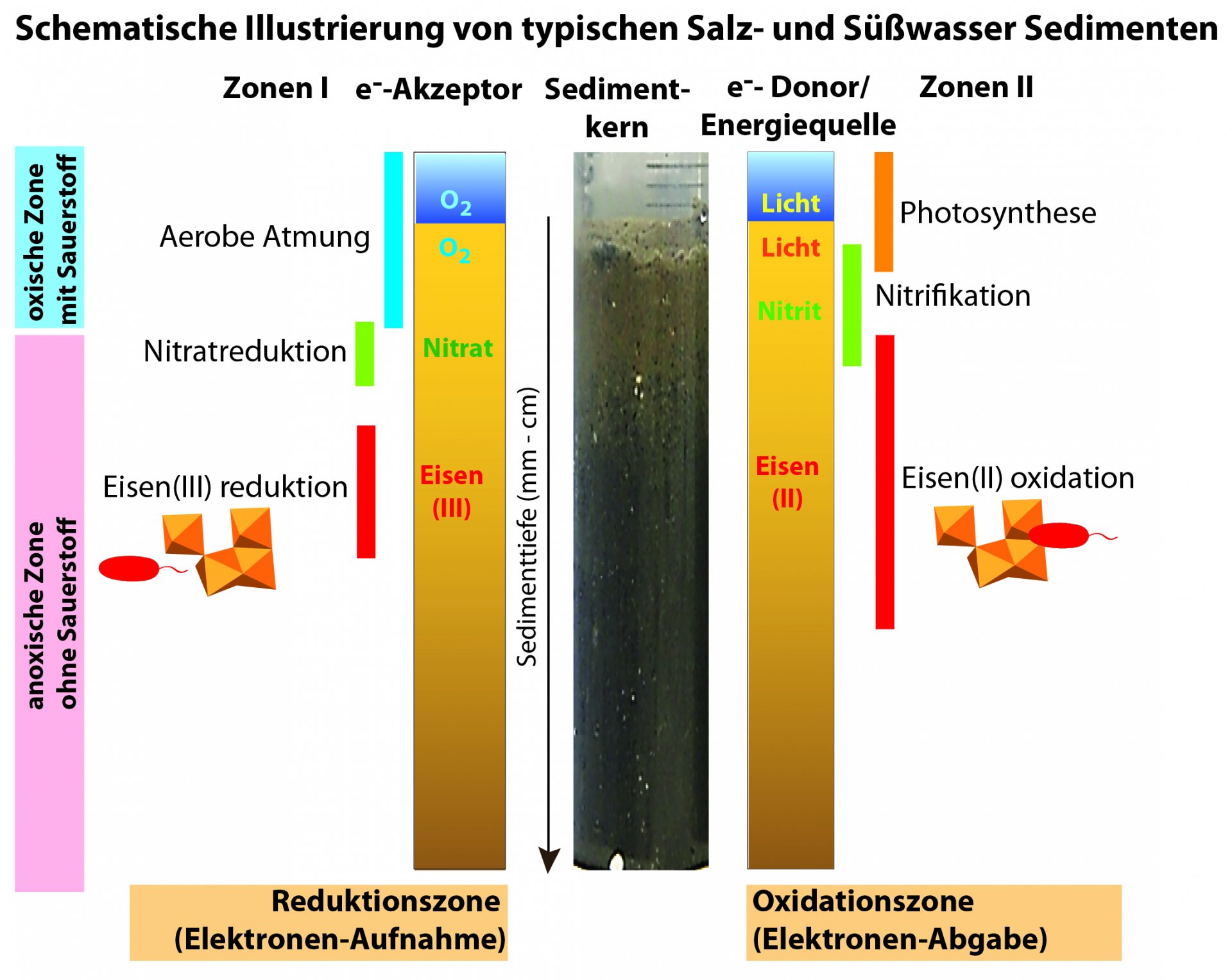
The biogeochemistry of coastal sediments is usually characterized by vertical gradients of electron donors and electron acceptors. During microbial conversion processes, the electron acceptors are consumed in a typical order. The degradation of organic compounds with O2 provides the highest amount of energy and therefore O2 is typically consumed first, followed by nitrate, iron(III) and finally CO2. Iron is after O2, silicon, and aluminium one of the most common elements on earth and is involved in a variety of metabolic and chemical processes. In order to generate energy for cellular processes and growth, FeB convert iron between iron(II) and iron(III). FeB are widespread in nature and contribute to all major biogeochemical cycles.
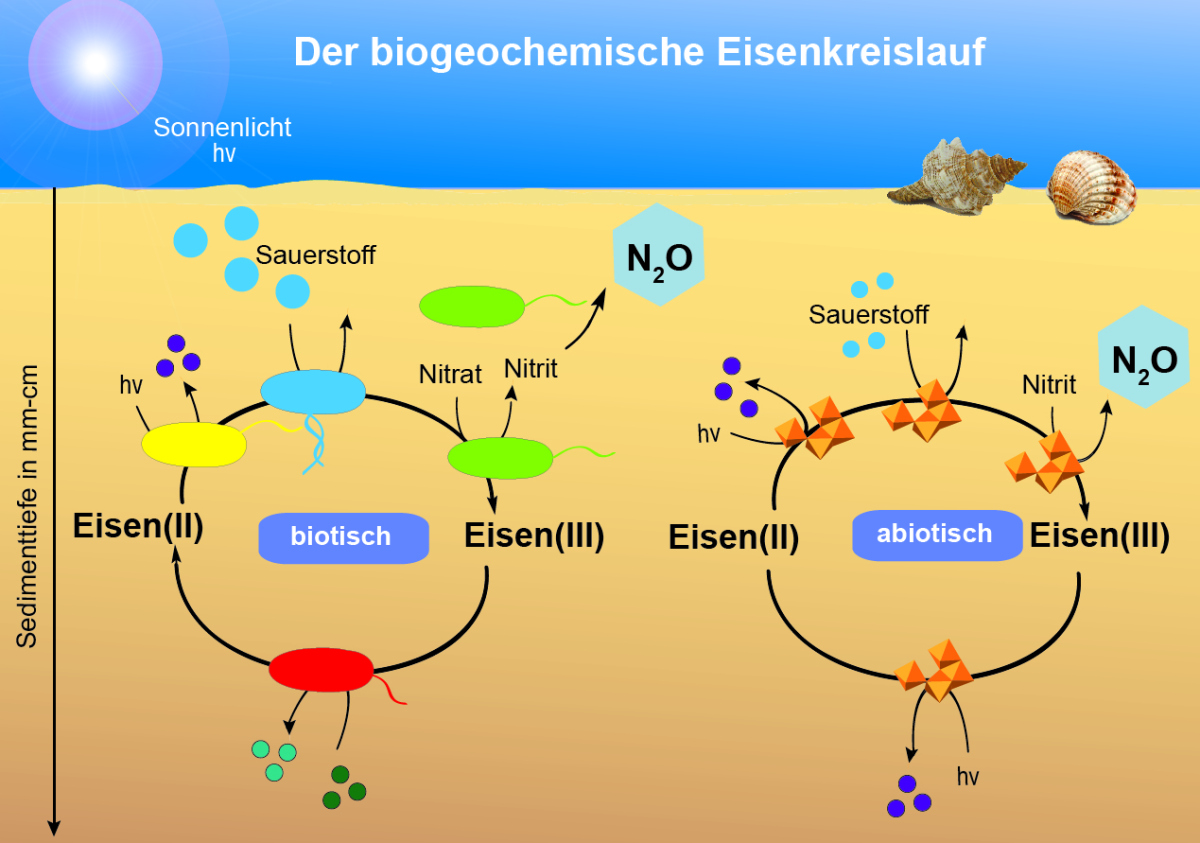
*See German webpage*
Unexpected distribution of iron-cycling microorganisms in coastal sediments
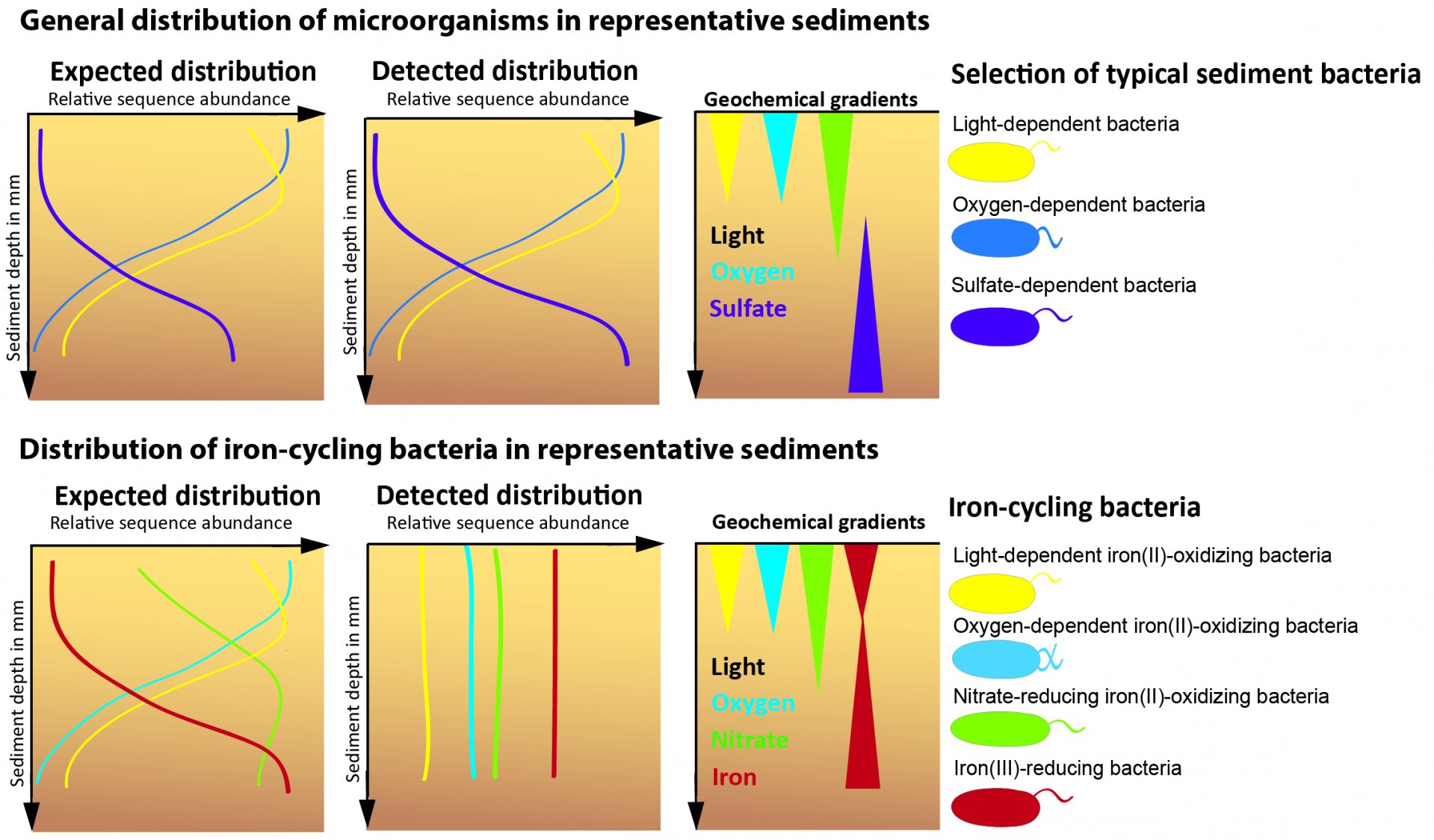
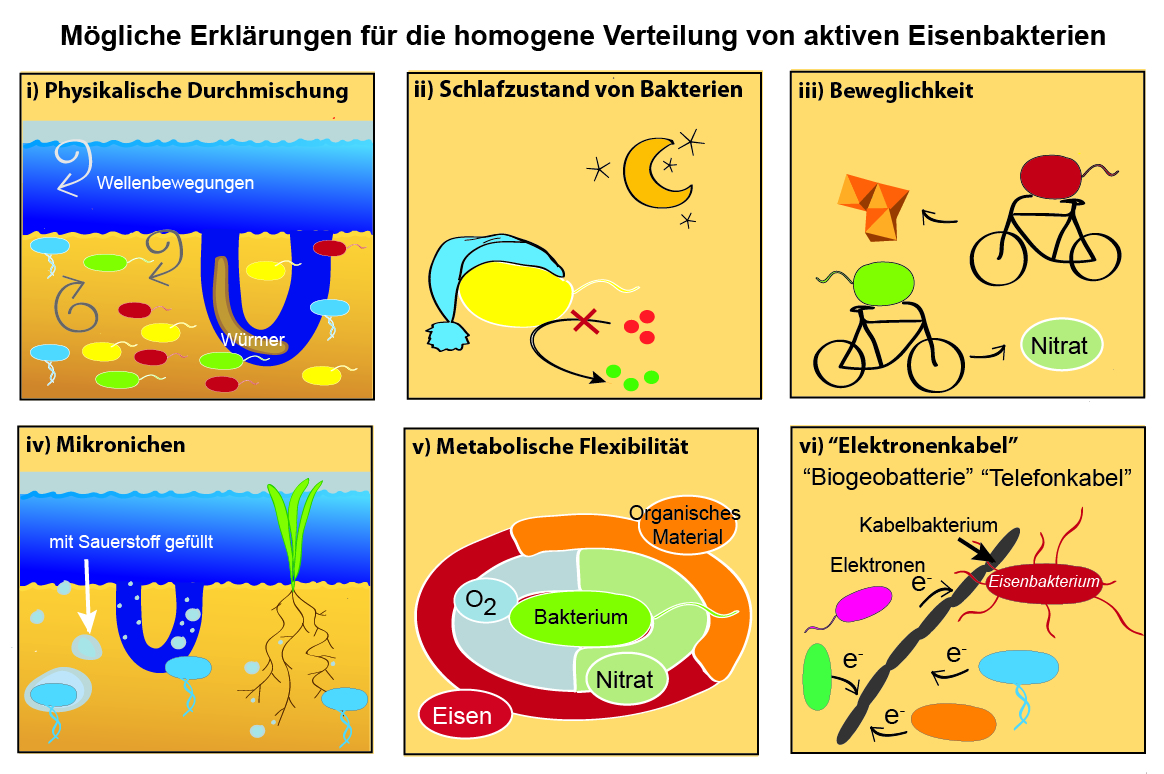
Until now, the natural distribution of active FeB in coastal sediments was unknown. As a graduate student in Andreas Kappler's and Sara Kleindienst's laboratory at the University of Tübingen, I investigated the diversity, distribution, and activity of FeB in representative sediments: freshwater sediments from Lake Constance (Germany) and marine sediments from Aarhus Bay (Denmark). The microbial community was characterized at the O2-rich and O2-poor interface of the upper 3 cm. Geochemical profiles of iron, O2, nitrate, and light showed a specific stratification of the investigated sediments. This stratification could explain 75-85% of the vertical distribution of all sediment bacteria (Figure 1). Therefore, such a distribution was also expected for FeB. Instead, a homogenous distribution of the active FeB was found in the investigated sediment depths, which means that the active FeB do not orient themselves by geochemical gradients. This behavior can be explained by the fact that the FeB i) are mobile and can "sleep" until better conditions are achieved, ii) live in microniches filled with O2 or nitrate, iii) can metabolize different substances, or iv) interact with the recently discovered cable bacteria (CB).
*See German webpage*
One potential explanation - the extraordinary microbial "Electro Party"
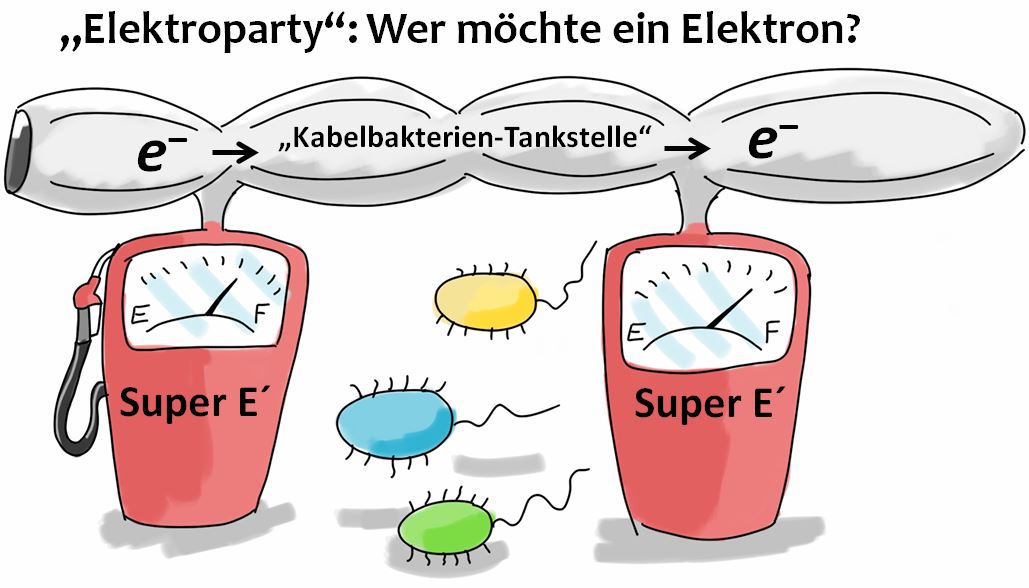
CB are able to transport electrons over long distances in sediments and indeed function like electric cables! With my sequencing data I could show for the first time that CB and FeB are both active in the same sediment layers. This means in detail: the iron(II)-oxidizing bacteria may be able to transfer electrons to the CB and the iron(III)-reducing bacteria can take up electrons with the help of the CB. Thus, the extraordinary "electrogenic connection" of CB and FeB could explain the special distribution pattern of "rocky" FeB in the investigated “hard rock” and “heavy metal”-containing sediments.
*See German webpage*
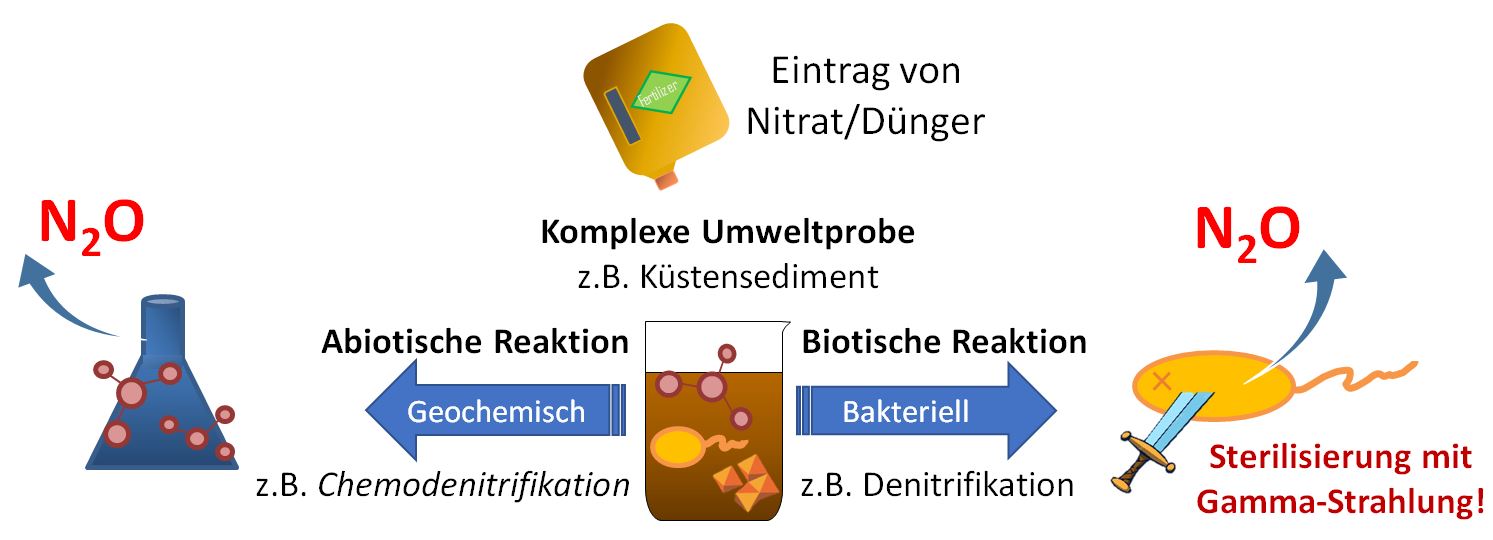
Geochemical activities in coastal sediments - the unknown contribution to the global N2O budget

CB and nitrate-degrading FeB - which are active in all investigated sediment layers - can use nitrate in the absence of O2 and thus contribute to the degradation of nitrate followed by nitrite and N2O formation. The homogeneous distribution patterns of CB and FeB also have a major influence on the surrounding geochemistry. Geochemical processes are also involved in the iron and nitrogen cycle. For example, the rapid abiotic reduction of nitrite by iron(II) can lead to an abiotic production of N2O (chemodenitrification). Until now, the specific contribution of chemodenitrification to the global N2O budget was unknown. With my experiments I was able to determine the N2O formation in natural (microbially + geochemically active) and sterile (only geochemically active) sediment. The comparison of natural and sterile sediment showed that a remarkable amount of at least 15-25% of the total N2O formation was caused by chemodenitrification, while 75-85% of the total N2O concentration was formed by microbial processes. This suggests that the process of chemodenitrification can contribute significantly to global N2O formation and also affect the microbial community.
Summary
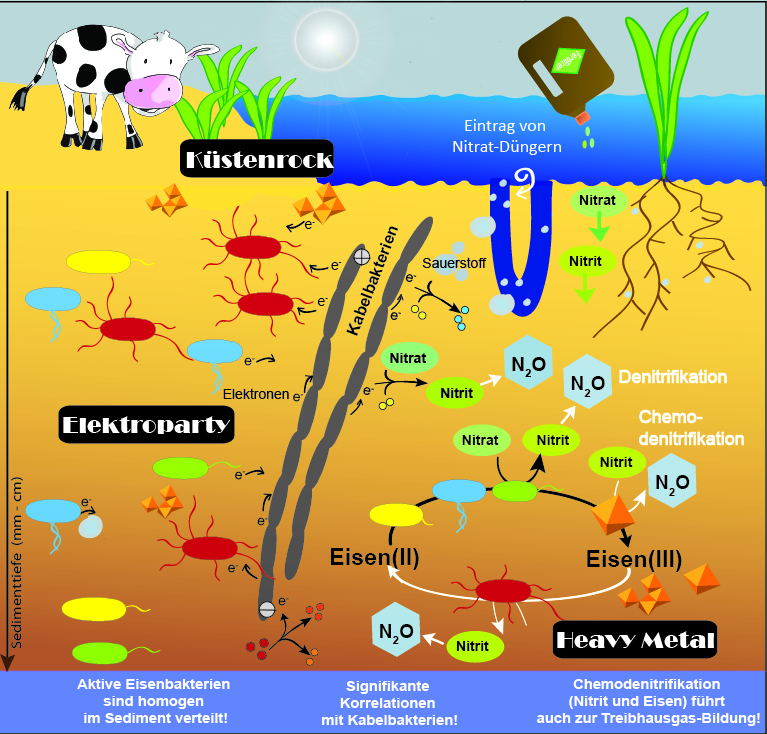
These new findings improve our understanding of bacterial and geochemical processes leading to N2O formation. Especially, in highly complex nonlinear contexts such as climate changes, it is important to identify all the relevant parameters and take them into account when we are developing climate models. We have a huge responsibility to future generations.