NanoSIMS 50L
Secondary ion mass spectrometry (SIMS) is a surface analysis technique. It provides information about the lateral distribution of any element and its isotopes as well as a quantitative information about the isotopic composition of a sample. Using a well focused ion beam the sample surface is bombarded with primary ions and secondary ions sputtered from the sample are analysed in a mass spectrometer.
The Max Planck Institute for Marine Microbiology finished the installation of a NanoSIMS 50L in March 2008. The funding for the acquisition of this instrument was provided by the Innovation Fund of the Max Planck Society.
The NanoSIMS 50L is equipped with Cs+ and O- primary ion sources, an electron gun for analysis of insulating samples, a secondary electron detector, and a magnetic sector mass analyser with a large version of the magnet and a multi-collection system of 7 detectors all equipped with Faraday cups and electron multiplier detectors. This configuration allows us the possiblity of analysing any mass from helium to uranium as well as molecules and clusters of atoms.
The NanoSIMS 50L is a nanometer scale secondary ion mass spectrometer with both extremely high lateral resolution and high mass resolution. Because of a unique ion optic design, the primary ion beam can be focused to a very small spot down to less than 50nm beam size. The high mass resolution of the mass analyser allows the separation of the isotope (mass) of interest from interferencing isotopes and/or molecular clusters with very close masses.
For more information on the unique capabilities of the NanoSIMS 50L in environmental microbiology applications, please visit our project pages.
Environmental microbiology applications
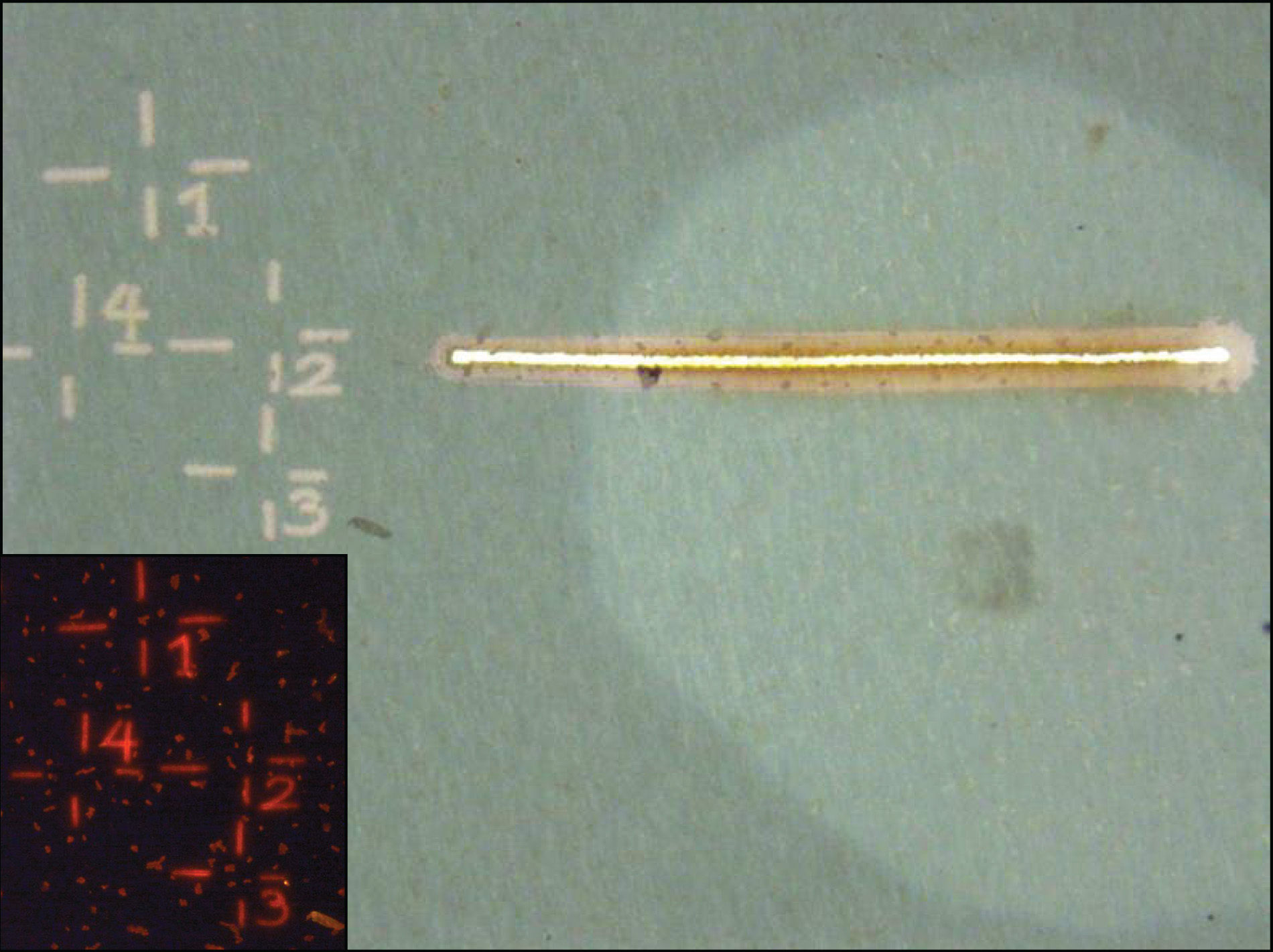
Prior to a nanoSIMS analysis, fields of view containing cells of interest are marked using the laser microdissection microscope (Leica LMD 6500). This facilitates sample orientation during nanoSIMS analysis.
The laser microdissection microscope (LMD) is equipped with various air objectives (10x - 63x) and fluorescent filters (i.e. DAPI, Cy3, Cy5, Alexa594) to identify, mark and cut out single cells using a UV laser (wave length: 355 nm, pulse frequency: 80 Hz, pulse length: <4 ns, avg. pulse energy 70 μJ). Imges of the marked areas are recorded using a CC7000 color camera.
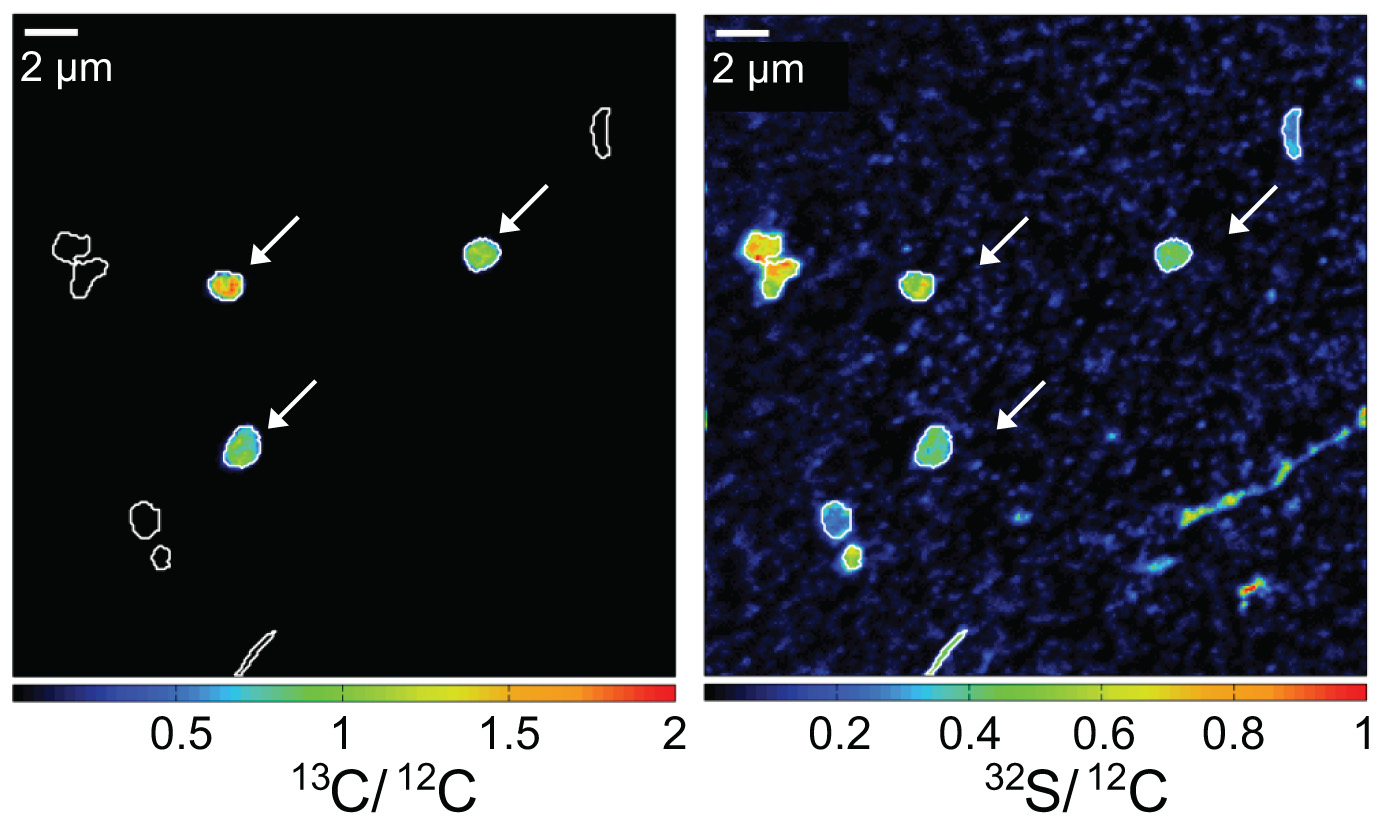
While oxidizing methane, aerobic methanotrophic bacteria also assimilate methane carbon into their biomass. When incubated in the presence of 13C-labeled methane, active methanotrophic bacteria can be identified by their high cellular 13C/12C ratios. In this case, a water column sample from a stratified Lake Cadagno was analyzed. Arrows indicate gamma-proteobacterial methanotrophs. Corresponding 13C/12C an 32S/12C nanoSIMS images show assimilation of 13C-labelled methane and the distribution of cell biomass, respectively.
Our current projects involving nanoSIMS for study of methane oxidation can be found HERE.
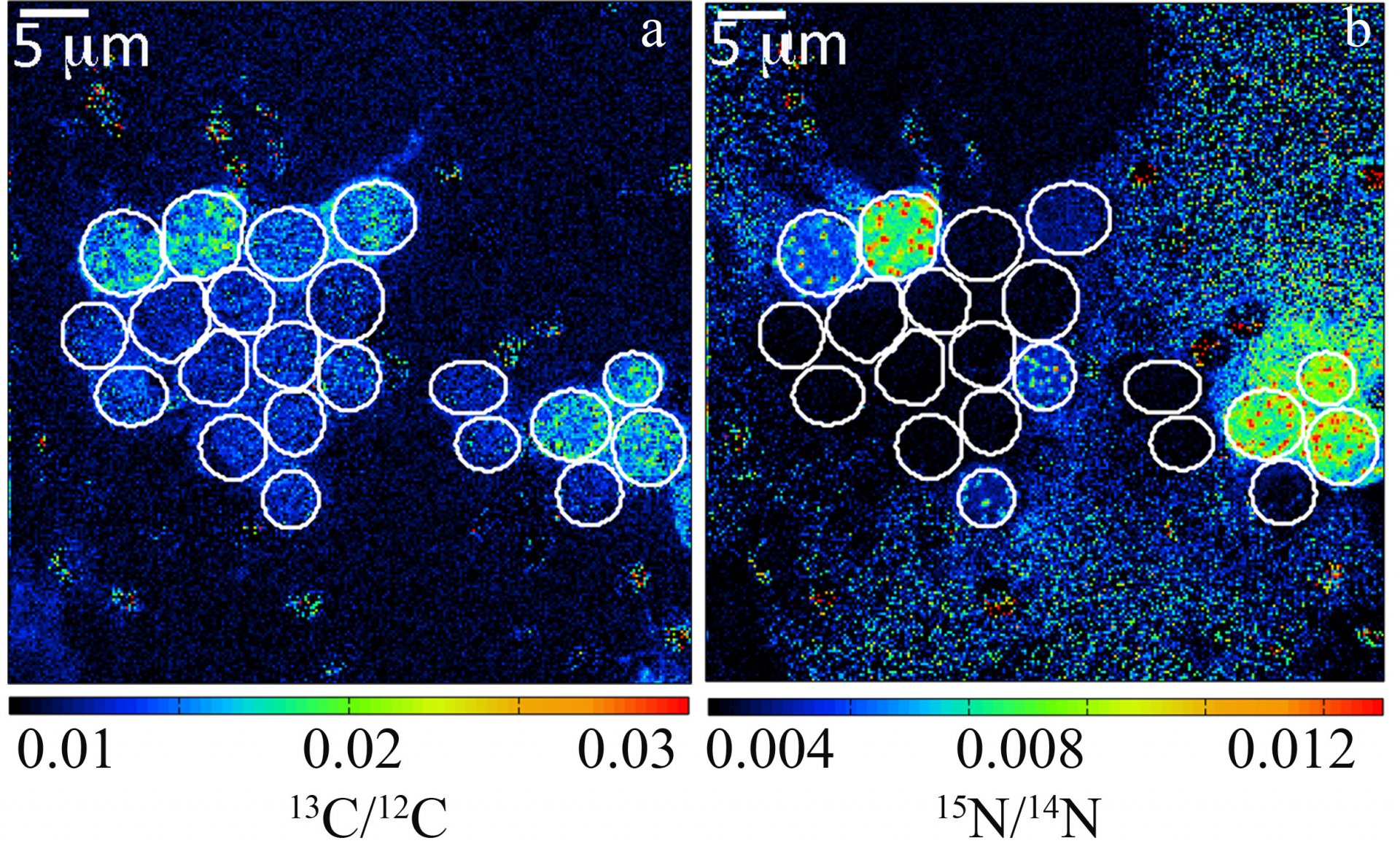
By recording the 12C15N/12C14N and 13C/12C ratios of individual cells, estimates of individual single-cell carbon and N2 fixation rates can be made. Here, carbon and N2 fixation rates of unicellular Crocosphaera watsonii-like cyanobacterial cells from the subtropical North Pacific Gyre were measured. These were one of the first direct in situ activity measurements of these important diazotrophs.
Our current projects involving nanoSIMS for study of nitrogen fixation can be found HERE.
Contact Information
Wissenschaftler/in
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Raum: |
3130 |
|
Telefon: |

Technikerin
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany
|
Raum: |
3131 |
|
Telefon: |
