- Presse
- MPI and MIT researchers prove fast m...
MPI and MIT researchers prove fast microbial evolutionary bursts exist
There are more than a dozen species of finches that evolved on the Galapagos Islands, each species identified by beak shape and size. Some have strong beaks to crack nuts while others have long and fine beaks to grasp larvae with surgical precision. All of the finches evolved from a common ancestor in a very short period of time, an evolutionary process known as adaptive radiation.
Although this burst of adaptive radiation is common for animals and plants, it has remained elusive and difficult to document in microbes in the wild -- until now.
New research published recently in the journal Nature Communications and led by Jan-Hendrik Hehemann, Philip Arevalo and Manoshi Datta from the Max Planck Institute for Marine Microbiology and the Massachusetts Institute of Technology (MIT CEE) shows that adaptive radiation does exist in microbes.

Microbial adaptation
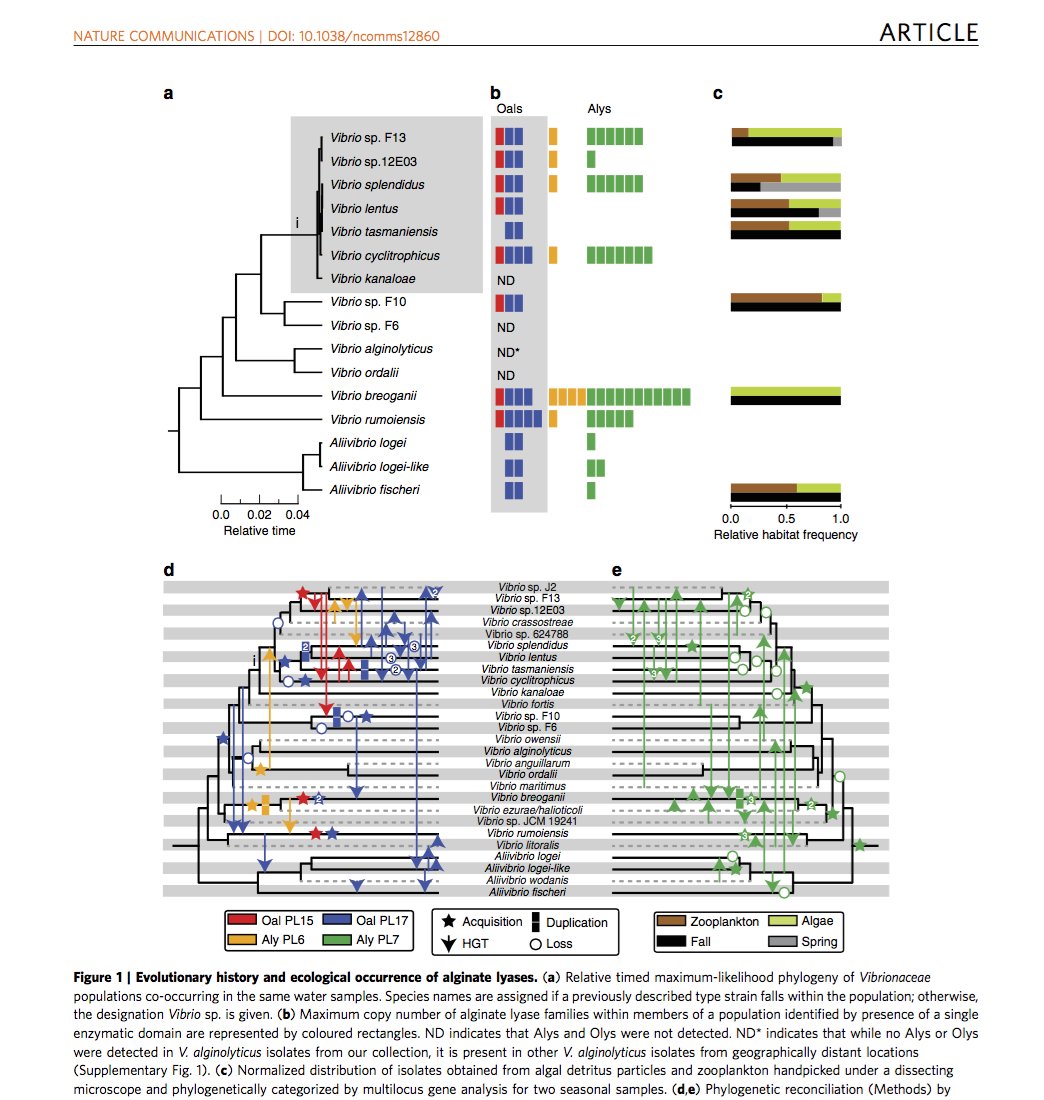
The scientists worked over several years observing ways in which a group of bacteria (known as Vibrionaceae) degraded cell walls of fast-growing marine seaweeds including kelps. They noticed that the microbes, while so closely related that most people would consider them a single species, were ecologically differentiated.
“We were able to reveal the evolution of different ways the bacteria consume alginate, an important carbohydrate from kelp,” said Hehemann, who sought a postdoc appointment at MIT CEE precisely to explore this question of how microbes adapt to different resource use.
Hehemann, now a group leader at the Max Planck Institute for Marine Microbiology in Germany, is a biochemist by training as well as a structural biologist. He found that closely related populations of bacteria had very different sets of enzymes suggesting they had different feeding strategies. Like the beaks of Darwin´s finches, these enzymes allow the bacteria to most efficiently consume a narrow set of resources derived from kelp.
“Many people doubted that microbial adaptation should be possible in contemporary environments since microbes have co-evolved with ecosystems on the planet for billions of years, so that most ecological opportunities might have been taken advantage of,”
- said MIT CEE Professor Martin Polz.
“We demonstrated adaptive radiations do happen and that they can lead to a rapid diversification of a single species into multiple, differentially adapted species.”“Microbial diversity is so vast, which is why it remains a major challenge to understand how they all fit into this world. However, our work shows that resources might be partitioned at much finer scales than we previously thought,” Polz said. “This may be part of the puzzle why there are so many microbial species.”
“One of the unique and powerful aspects of this project is that we were able to generate a hypothesis by looking at bacterial genomic data, test their metabolic capabilities in the lab, and then go back to the genomes to see how these traits evolved,” said Datta. She is a computational biologist and helped bridge Hehemann’s lab work with Arevalo’s computation analysis.
Gene transfers
Arevalo explained the team looked at hundreds of Vibrionaceae strains whose full genomes were genetically sequenced. “What I was doing was computational -- finding how the microbes adapted to different forms on the algae, and then looking at the history of these microbial genes.”
Through this analysis, the researchers found that horizontal gene transfer, not point mutation, was the primary diversification driver. Horizontal gene transfer, by definition, is a process by which an organism receives genetic material from a source other than its parent (e.g., from neighboring cells or the surrounding environment). In principle, this could occur through numerous mechanisms, which are still being investigated, and can transfer genes among organisms that are not closely related.
“It’s very common to have gene duplication in bacteria, presumably to be able to synthesize a particular enzyme faster,” commented Polz. “This research shows, however, that these bacteria duplicate not just one copy in the same genome, but instead acquire a new copy of the same functional gene by horizontal gene transfer from one microorganism to another. This differentiation tells us how selection in the environment works to create different types of organisms that either work together when degrading a substrate or compete when seeking nourishment.”
“It takes a village,” he said.
The discovery could portend new applications that create more economical and renewable biofuels, or innovate biomedical chemicals and products.
Reprinted with permission of MIT News.
Original publication
Please direct your queries to …
Phone: +49 421 218 65775
E-Mail: [Bitte aktivieren Sie Javascript]
or the press office
Dr. Fanni Aspetsberger
Dr. Manfred Schlösser
Phone: +49 421 2028 704
E-Mail: [Bitte aktivieren Sie Javascript]